Stablepharma reveals 12 months stability data for our second fridge-free vaccine, SPVX06 for Tetanus.
This significant milestone reinforces Stablepharma’s ability to demonstrate the robustness of our platform of reformulated vaccines, including SPVX02 for the prevention of Tetanus diphtheria. In-vivo challenge studies demonstrated that SPVX06 maintains its potency, providing comprehensive protection while effectively stimulating the immune system to produce antibodies.
The reformulated Tetatox vaccine, a Tetanus Toxoid (TT) vaccine manufactured by Bulbio Ltd (BB-NCIPD), was converted into SPVX06 (TT) lyophilized in single-dose vials, has shown enhanced thermostability when stored for one year at +40°C/75%RH. Tetanus Toxoid (TT) is normally stored at 2 – 8 °C in a refrigerator.
The results indicate that SPVX06 provides full protection and sustains immunogenicity over time, exceeding the minimum potency required by the World Health Organization (WHO) for releasing Tetanus vaccines (see below).
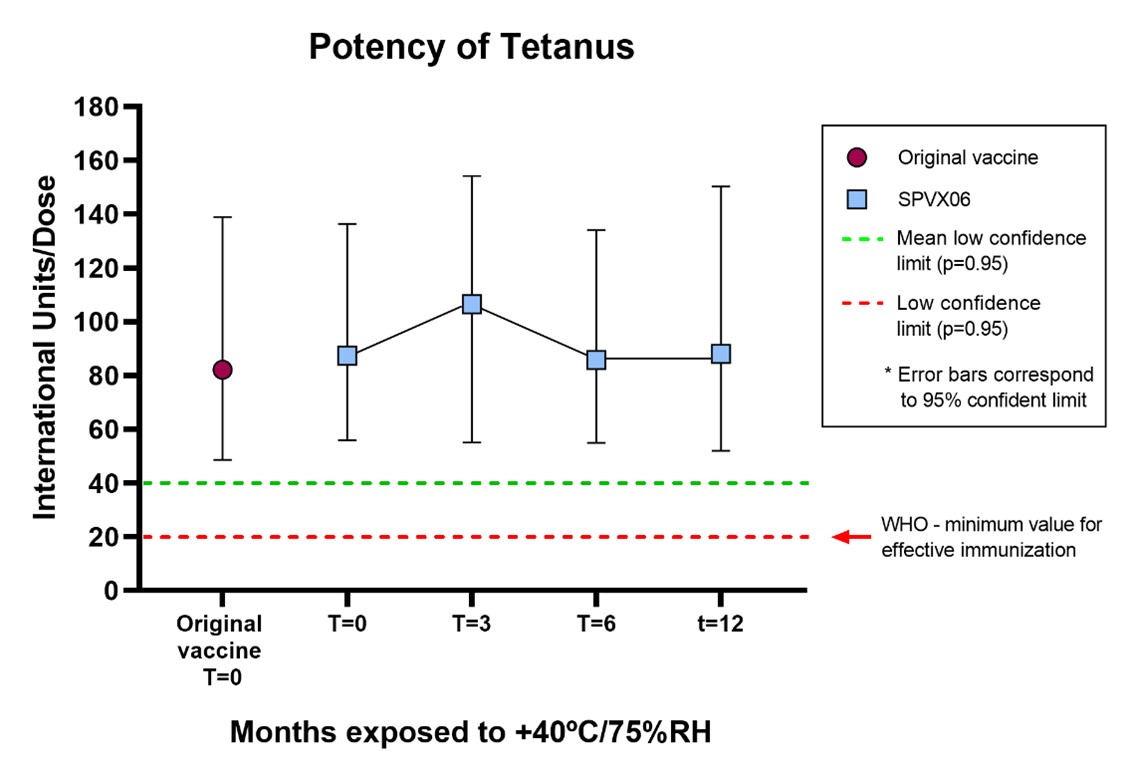
The graph summarises the stability profile of SPVX06 in terms of potency. Each point is calculated by comparing SPVX06 with a reference vaccine calibrated in International Units following the same EU Pharmacopeial Potency assay BulBio uses for batch release of their Tetatox vaccine.
The SPVX06 project aligns with our commitment to positively impact global health, and we aim to follow a similar model, through partnerships with other European vaccine manufacturers to expand our portfolio of vaccines.
