Our founder, Dr Bruce Roser’s extensive research over decades led to the discovery of a novel technology that eliminates the need for the global cold chain.
As a result of this discovery, we are launching the world’s first fridge-free vaccines for the treatment and prevention of serious diseases.
Up to 50% of global vaccines are wasted due to failures in the cold chain according to the WHOStablevaX™ technology is a novel and unique approach to making fridge-free vaccines a reality
The Stablepharma Story
In his early career, Stablepharma’s founder Dr Bruce Roser MB BS, PhD identified and patented the process of using Trehalose to achieve a state of ‘suspended animation’ in a sugar glass, to thermally stabilise a range of vaccines. Trehalose is a naturally occurring sugar with remarkable chemical stability that can stabilise delicate molecules against very hostile environments.
Trehalose is now commonly used in many pharmaceutical products worldwide and is observed naturally in some living organisms, most notably in the case of the “resurrection” plant.
Over the years, Dr Bruce Roser’s R&D programmes continued, and he established Quadrant Bioresources, (later renamed Quadrant Healthcare). Quadrant, together with its IPR, was purchased by Elan Pharmaceuticals for over £50m in 2001.
Dr Bruce Roser and Dr. Arcadio Garcia De Castro, our Chief Scientific Officer (CSO) have worked together for decades and between them, they have over 70 years of experience stabilising pharmaceutical products, including vaccines. Stablepharma’s technology platform StablevaX™, reformulates existing approved vaccines, enabling distribution and storage, in even the most inhospitable parts of the world.
StablevaX™ provides a novel and unique approach to making fridge-free vaccines a reality and reduces the need for the cold chain (refrigeration). https://stablepharma.com/new-data-shows-stablevax-td-stable-for-12-months/
What motivates us
The problem
Vaccines are thermally unstable products that lose their effectiveness if exposed to temperatures which are either too hot or too cold. Most vaccines require constant refrigeration between 2-8°C. COVID mRNA vaccines require even lower temperature cold chain – up to -80°C. The World Health Organisation (WHO) estimates that 50% of the world’s vaccines are rendered useless each year due to failings of the existing refrigeration processes (known as the ‘The Cold Chain’) used for delivery and storage.
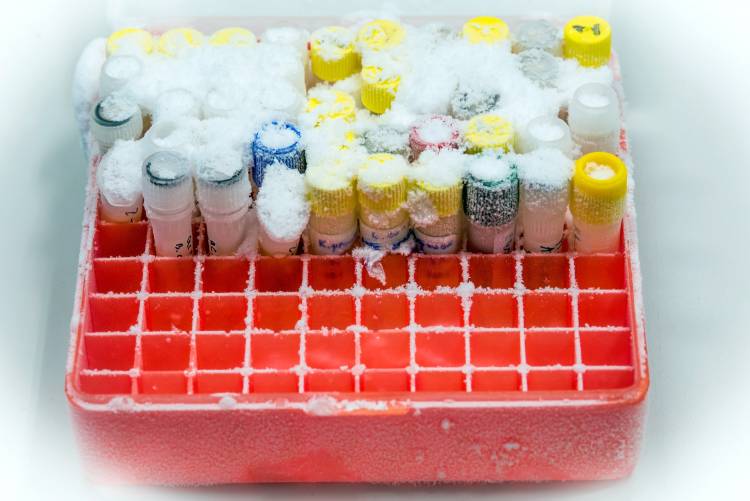
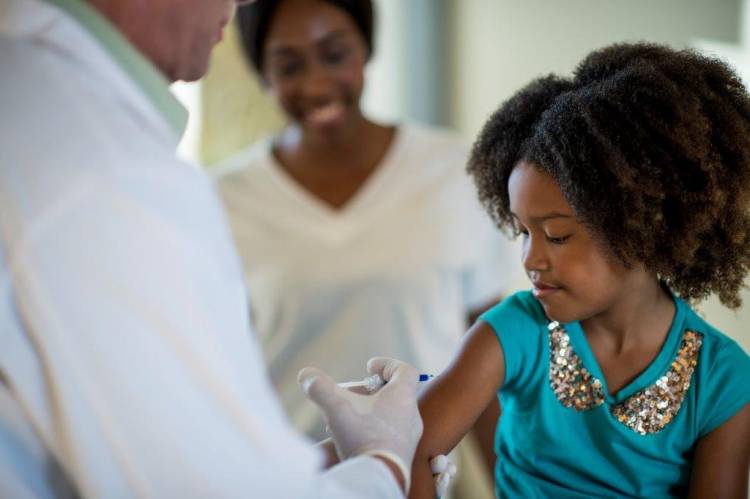
The human cost
There are 3 million child deaths per annum that are currently prevented by vaccination – we want to help vastly improve this number. The “Cold Chain” failure reduces the effectiveness of vaccination programmes, and this can lead to unnecessary deaths and the possible re-emergence of disease.
StablevaX™ will extend the shelf life of most vaccines at high temperatures, allowing for safe distribution and storage, for many years, without loss of potency.
The economic cost
The World Health Organization reports that over 50% of vaccines are wasted around the world each year. Given global vaccines sales of $60 billion in 2020, wastage equates to $30 billion per year.
The global cost of the ‘Cold Chain’ is estimated at $ 400m per annum. It is unreliable, expensive and fails frequently.
Stablepharma’s novel StablevaX™ technology could reduce costs dramatically while improving global health outcomes.


The carbon footprint
The storage and cold chain supply of vaccines has caused a significant environmental impact. For example, 541.71 million doses of COVID-19 mRNA vaccine administered in the United States of America equates to 595,881 kg of Co2. This is equivalent to over 2 million miles in an average petrol car or over 2,100 economy class return flights from London to Berlin.
Stablepharma aims to lower the impact of vaccine distribution and storage on the environment by reducing the need for the cold chain, through the development of fridge-free vaccines.
The Shareholders View of Stablepharma
We use our StablevaX™ technology to reformulate and thermo-stabilise existing and new vaccines in partnership with vaccine manufacturers. Through this, we reduce the need for the cold-chain, reduce wastage, costs and carbon emissions. To find out more:
