Stablepharma and BB-NCIPD Ltd (Bul Bio) establish thermostability of StablevaX™-Td Vaccine After Exposure to +45°C for 12 months
In-vitro and In-vivo Studies Pave the Way for Fridge-Free Vaccines
Stablepharma Ltd and BB-NCIPD Ltd (Bul Bio) formed a strategic partnership in 2021 to produce the world’s first fridge-free Tetanus diphtheria (Td) vaccine, StablevaX™-Td. Encouraging potency and accelerated thermo-stability data have been reported.
“Since we formed our partnership with European vaccine manufacture Bul Bio, our aim has been to produce the world’s first fridge-free Td vaccine and we are making outstanding progress” said Özgür Tuncer, CEO & Executive Director Stablepharma Ltd.
Methods
To establish the thermostability of StablevaX™-Td, in-vitro and in-vivo tests were conducted.
In-vitro ELISA (Enzyme Linked Immunoassay) tests were utilised to determine the integrity of tetanus and diphtheria toxoids of samples stored at +45ºC.
Aluminium hydroxide is an adjuvant present in the standard liquid Td vaccines (i.e. a substance that increases the immune response). When vaccines are exposed to freezing or high temperatures, aluminium adjuvant is damaged, compromising the efficacy of vaccines. Aluminium hydroxide test were performed on StablevaX™-Td to verify the stability and the integrity of the adjuvant was maintained.
Most recently, Stablepharma and Bul Bio concluded in-vivo animal challenge studies (i.e. potency tests in animals) for the Tetanus component of the fridge-free Td vaccine, StablevaX™-Td. The studies aim to assess, according to European Pharmacopeia 2.7.8. and Bul Bio SOP-QC005, the product’s ability to induce a protective immune response in suitable animal models.
Potency is determined by administration of the Tetanus vaccine to animals (guineapigs) followed by challenge with tetanus toxin (i.e. administration of toxin into animals). It is calculated by comparison of potency to the reference vaccine, calibrated in International Units (IU). Bul Bio have conducted the tests in compliance with their standard batch release procedures.
Accelerated product stability is tested by keeping StablevaX™-Td and the reference vaccine at +45°C for time intervals of t=0, 3, 5, 10 and 12 months.
Three different StablevaX™-Td formulations were tested over 12 months: StablevaX01 (pre-filled syringe with dry vaccine on a medical sponge), and StablevaX03 and StablevaX04 (pre-filled syringes with immediately dissolvable dry powders), all diluted with Water For Injection (WFI) prior to injection.
Results
Analytical results through ELISA tests established the integrity of StablevaX™-Td. ELISA assays showed that the toxoids in the reference vaccine were damaged by 1 month at +45°C and undetectable by 10 months. Toxoids were detectable in StablevaX™-Td formulations which were kept at +45°C for up to 14 months.
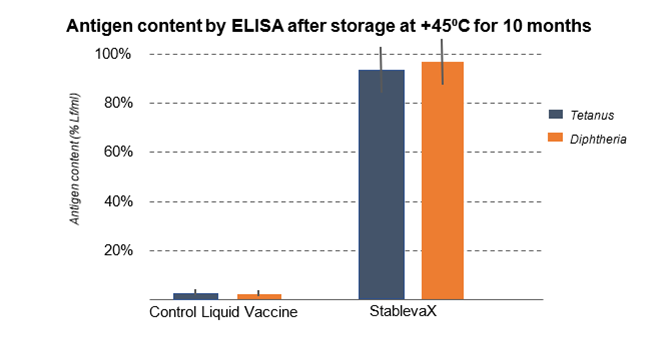
StablevaX™-Td formulations which were kept at +45°C for up to 14 months.
Graph 1: In-vitro ELISA test conducted by Stablepharma comparing the tetanus and diphtheria components in a standard vaccine against StablevaX Technologies VX01/02/03
The effect of aluminium hydroxide was also maintained. When exposed to -20°C freezing temperatures or exposure to +45°C for 10 months, StablevaX™-Td maintained a similar structure to the reference vaccine stored at typical refrigeration temperatures. Conversely, the reference vaccine when exposed to the same temperatures aggregated (i.e. became unsuitable for use).
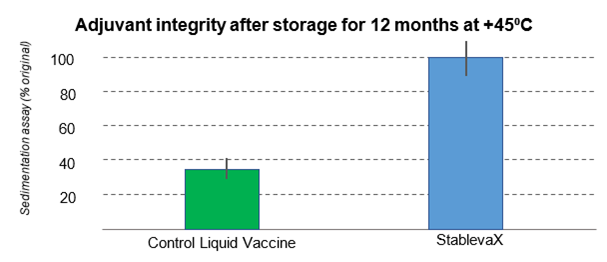
Graph 2: In-vitro adjuvant integrity test in a standard vaccine against StablevaX technologies.
The animal potency challenge test results demonstrated that the tetanus component of the StablevaX™-Td vaccine remains thermostable and as potent as the reference vaccine, after exposure to +45°C for 12 months. In contrast, the reference vaccine’s potency has deteriorated over time, dropping to zero after exposure to +45°C for 10 months.
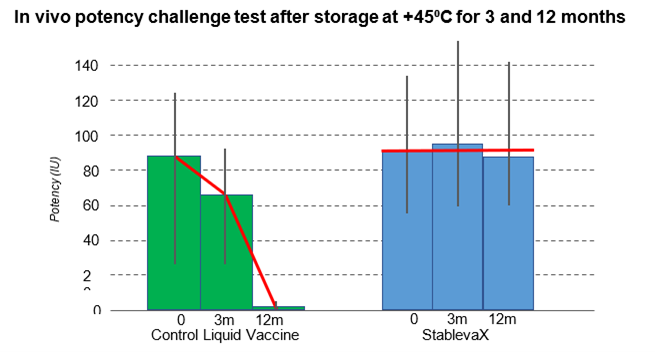
Graph 3: In-vivo potency challenge test for tetanus component in a standard vaccine against StablevaX™ technologies. Tests were conducted blind for Stablepharma Ltd. by GLP/GMP certified laboratory.
Conclusion
To be considered a fridge-free finished pharmaceutical product (FPP), World Health Organisation (WHO) stability guidelines outline the need for accelerated thermostability at +40°C ± 2°C/75% RH ± 5% RH for a minimum time period of 6 months at submission and a long-term stability at +25°C ± 2°C/60% RH ± 5% RH or +30°C ± 2°C/65% RH ± 5% RH or +30°C ± 2°C/75% RH ± 5% RH for a minimum of 12 months depending on the climate zone.
Based on the WHO stability guidelines, the StablevaX™ formulations developed for Td have the accelerated stability temperature requirements to be considered as fridge-free pharmaceutical products, not needing refrigeration.
