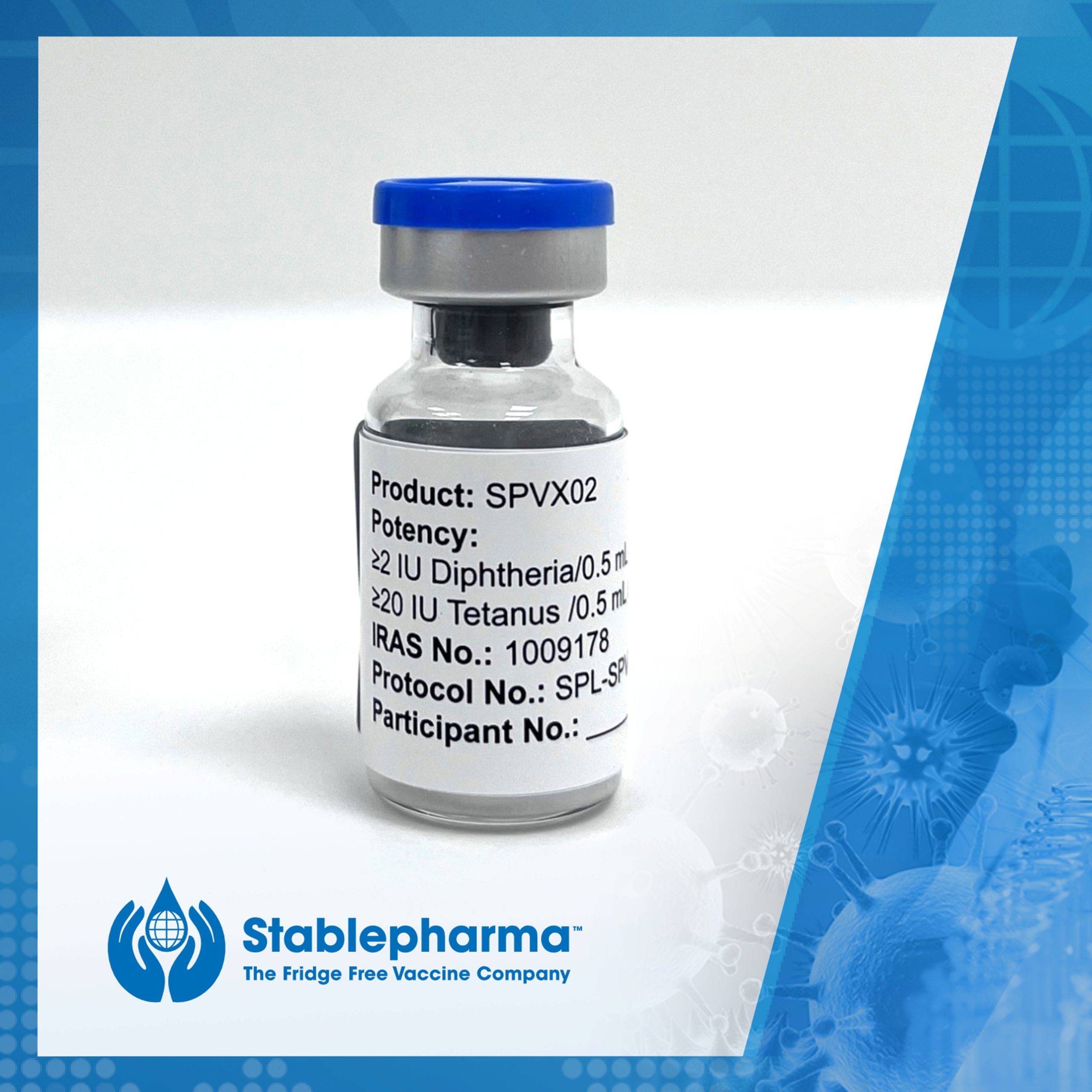
Following a number of ‘firsts’ for Stablepharma during 2024, we spoke with Ozgur Tuncer, CEO & Executive Director about a year that has accelerated the commercialisation of the world’s first fridge-free vaccine, SPVX02, for the prevention of Tetanus and Diphtheria. Ozgur also highlights some of the global challenges associated with the cold chain and talks […]
Read More