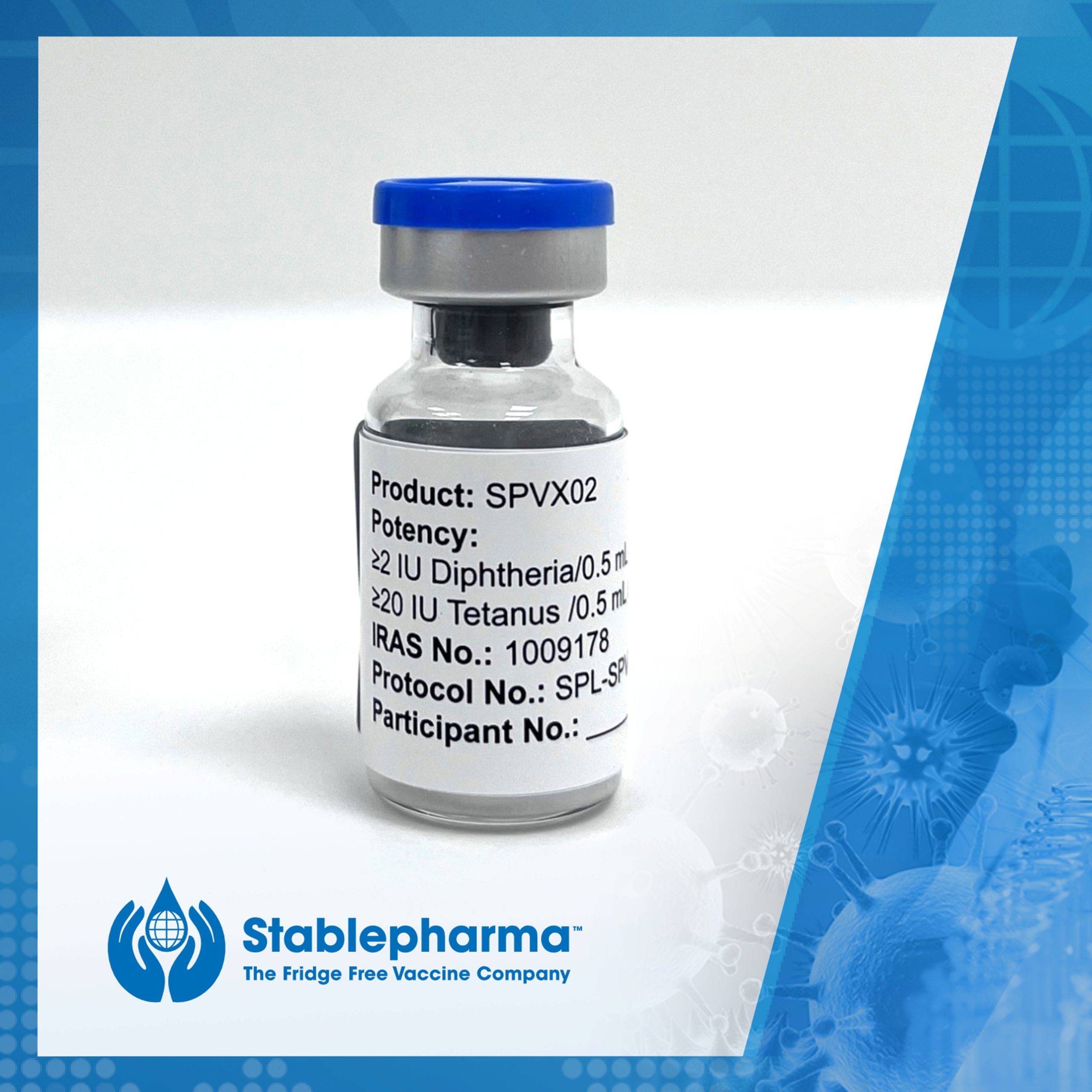
Stablepharma’s Fridge-Free Vaccine that Eliminates the Need for Cold Chain entering First-in-Human UK Clinical Trial. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted approval for Stablepharma to begin a Phase I clinical trial with SPVX02, an adult booster vaccine aimed at preventing tetanus and diphtheria (Td) infections. This approval marks a major […]
Read More